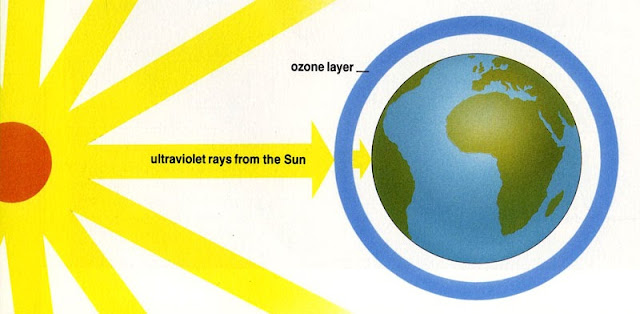
The ozone layer blocks a large part of the sun's rays and prevents too much ultraviolet radiation from reaching the earth. This is vital, because the high-energy ultraviolet rays of the sun can destroy the cells of animals and plants and damage human skin. If the ozone layer in the stratosphere is intact, it acts like a huge protective screen against aggressive UV radiation.
The ozone layer in the atmosphere protects the earth's surface from dangerous radiation. However, this protective shield is endangered by chemical compounds, especially CFCs from refrigerators and spray cans.
Sunlight and Ultraviolet (UV) Radiation
Sunlight is essential for human health because, among other things, it regulates the daily and annual rhythm and helps supply the body with vitamin D.
However, if you expose your body to excessive solar radiation, damage to health, which is mainly caused by the shorter wavelength ranges, can occur. These include skin cancer, skin aging, eye diseases and the weakening of the immune system.
The skin protects itself from UV radiation by storing the complex molecule melanin. With longer and more intensive radiation, the skin becomes reddened by the high energy of UV-B radiation and sunburn occurs. As the disease progresses, severe burns and blistering occur and the tissue dies. As a result, more often Long exposure to UV radiation results in irreversible changes in the skin such as wrinkles and permanent vasodilatation.
The most serious long-term consequence is skin cancer, which is caused by changes in the genetic make-up in the skin cells caused by UV-B.
“
Ultraviolet radiation is divided into three wavelength ranges according to its biological effect.
- UV-A radiation - 315–400 nm (UV-A radiation and visible light hit the earth's surface practically unfiltered)
- UV-B radiation - 280-315 nm (more energetic, dangerous - Ozone and oxygen molecules absorb over 90 percent of the incident UV-B radiation)
- UV-C radiation - 190–280 nm (Highly energetic radiation, completely absorbed by ozone and oxygen molecules in the stratosphere and therefore does not reach the earth's surface).
How is ozone formed in the atmosphere?
Ozone is a natural component of the earth's atmosphere, but only occurs in very low concentrations. Near the ground it reaches a proportion of around 30 molecules in one billion air molecules. However, the ozone concentration in the stratosphere increases significantly with altitude. The maximum of the ozone layer is measured in the middle stratosphere in an altitude range between 15 and 30 kilometers, where the mixing ratio of ozone to air can be 300 times the value close to the ground.
Stratospheric ozone is formed naturally in photochemical reactions with the participation of molecular oxygen and ultraviolet solar radiation.
Photochemical ozone production is most effective where the sun shines longest and most intensely, in the tropics and subtropics. From there the ozone is transported with the large-scale circulation to higher geographical latitudes.
The ozone is formed in the stratosphere from molecular oxygen in two steps: In the first step, diatomic oxygen molecules O2 are split into two oxygen atoms each by the sun's UV radiation. These are very reactive. In the second step, these oxygen atoms react with oxygen molecules to form three-atom ozone O3. The energy released in the process has to be absorbed by a third reaction partner (usually nitrogen or oxygen) by collision.
The stratospheric ozone not only represents an effective UV-B protective shield, but also releases the absorbed solar energy in the form of heat to the surrounding atmosphere. In this way, ozone significantly determines the vertical temperature structure of the stratosphere and thus also shapes the large-scale air currents.
What is causing the ozone hole?
If the ozone layer didn't exist, the sun's rays would literally roast us on the ground. Life in its current form would not be possible at all without this protective screen.
But the ozone layer has dangerously large holes. The reason for this are chemical substances that humans have blown into the atmosphere. The greatest enemy of ozone is chlorofluorocarbons, or CFCs. For a long time this gas was used as a coolant in refrigerators and as a propellant in spray cans. Itself odorless and non-toxic, CFCs are only dangerous to humans when they rise in the stratosphere, reacting with ozone, thus attacking and destroying the ozone layer.
Because of their inertia, they were considered harmless substances for a long time. In fact, CFCs do not pose a threat in the troposphere, but because of their extreme longevity, they can slowly make their way into the stratosphere above. In the stratosphere, the higher intensity of high-energy UV radiation then breaks down the CFC molecules. The chlorine released in this way then acts as a catalyst in destroying the ozone molecules.
Who discovered the ozone hole?
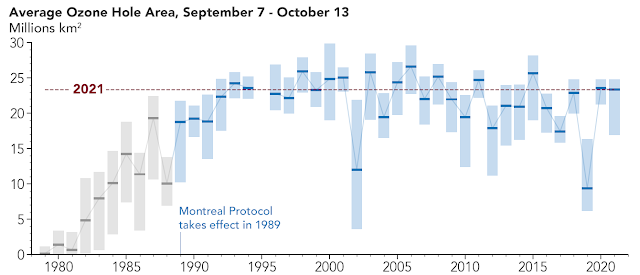
In 1985 a research team led by the British geophysicist Joseph Charles Farman, documented a drastic and progressive decrease in the ozone layer of up to 60 percent over the Antarctic. This phenomenon is known as the ozone hole and resulted with serious international negotiations on the issue of emission control.
Strong chemical ozone depletion is also observed in the arctic polar regions in spring. The ozone losses there are typically 20-25 percent, but can sometimes increase to up to 60 percent if the stratospheric temperatures are extremely low. Lesser but significant stratospheric ozone depletion is observed today over numerous other, also more densely populated areas of the earth.
What is being done to repair the ozone layer?
To protect the ozone layer, the Montreal Protocol was passed in 1987, an international agreement that came into force in 1989 and with which the signatory states committed themselves to a gradual reduction and finally to the complete abolition of the emission of chemicals containing chlorine and bromine that destroy stratospheric ozone.
The ozone-depleting substances are increasingly being replaced by fluorocarbons (PFCs). Although these HFCs protect the ozone layer, they are not climate-friendly. Depending on their structure, they contribute to global warming for each amount of gas introduced than the most important greenhouse gas carbon dioxide (CO2).
Photo: NASA.gov...The increasing warming of the troposphere simultaneously leads to a decrease in temperature in the stratosphere, which in turn accelerates the chemical ozone depletion. In addition to nitrous oxide, methane and, of course, carbon dioxide, PFCs are among the emissions that are to be monitored for climate protection under the Kyoto Protocol.
Since many processes, such as the connection between climate change and ozone depletion, are still largely not understood, it is difficult for scientists to make reliable predictions for the future of ozone depletion or build-up.